Ventilating Obstructed Lungs
There are two major references for the data contained in this post which have been linked here:
A summary of the ventilation of the patient with obstructive lung disease patient is below:
Setting up the ventilator for the Obstructed Lung

The problem with obstructive lungs that need ventilation
We often think of asthma (and COPD) as a problem of getting air out, however by the time the patient presents with a need for invasive airway management and ventilation, the problem also becomes one of getting air in.
One of the big challenges with ventilating the patient with obstructive pathology, is that simply placing an ETT and placing the patient on mechanical ventilation does nothing to help the patient, in fact, forcing more air, under more pressure is more likely to increase the danger to the patient and possibly worsen the condition.
OK, so what do I do then?
Let’s say we do get to the point where the patient with an obstructive lung pathology needs to be ventilated… first of all, what does this look like?
- Patient who is starting to tire with the massively increased work of breathing to move adequate tidal volumes
- Patient who is starting to not move adequate tidal volumes
- Increasing CO2 levels on ABG (or even on ETCO2 if you have been monitoring this throughout)
- Severe cyanosis and signs of hypoxia (PaO2 < 60mmHg or trending down despite maximal therapy)
- Level of consciousness starts to decrease
- Silent chest (despite maximal therapy) and respiratory arrest would be good indicators that you are not winning, but also may be a bit late.
If the above symptoms progress despite maximal therapy, then the patient may need to be escalated to airway management and invasive ventilation.
There are some things you should already have considered and have already tried to prevent this downward spiral of trapping of air and increasing pressure:
- Oxygen administered preferably humidified (increasing flow to meet oxygen demand) but be careful of administering oxygen and causing hyperoxia (see notes below)
- Nebulized Beta 2 stimulants and Ipratropium Bromide (ongoing and continuous if needed)
- IV/Oral or IM Steroids (corticosteroids)
- Magnesium Sulphate IV infusion
- Adrenalin subcutaneously in the case that the patient is getting worse despite therapy (or first line in the case of a silent chest)
- Consider IV Beta2 Stimulants (being careful not to delay other more effective treatments
The fix here is NOT to push more air into the chest, rather to ventilate in a way that limits the pressure buildup within the chest, and thus decreases the risk of hypotension, hypoxia and death. The whole aim of intubating this patient for ventilation is to maximize the expiatory time and decrease the pressure buildup within the thorax, to buy time for the medications you are still administering to work.
The aims of mechanical ventilation are:
- to decrease the work of breathing
- maintain oxygenation
- assist alveolar ventilation without causing more harm
Let’s have a look at the physiology and pathophysiology associated with the acute exacerbation of asthma/COPD leading to air trapping (this is a fairly complicated explanation that is summarized below).
How does a normal lung work to inflate and deflate?
Normally, the brain senses an increase in the concentration of CO2 in the system through chemoreceptors located in the arch of the aorta, the carotid bodies, and measuring CSF at the medulla oblongata.
This increase leads to stimulation of the respiratory center, increasing rate and volume of ventilation (to increase the minute volume).
Messages are sent down the phrenic nerve (that innervates the diaphragm) and other nerves that innervate the intercostal muscles to contract these muscles, making the volume inside the thorax larger, and the pressure inside the thorax lower.
This causes a rush of air into the lungs from the outside, in an attempt to equalize the pressure, and the lungs fill with air. As can be seen this is an active process as the pressure within the thorax needs to be decreased by increasing the volume of space within to allow for air movement.
In the patient who is trapping air, this is really difficult as the resting pressure within the thorax is already a lot higher when compared to the normal patient above, this results in a resting positive pressure within the thorax. In order to get air to move, this patient still needs to create a negative pressure but because their starting point is much higher, the patient needs to work much harder to move the same amount of air.
Often the patient with bronchospasm or air trapping will have a prolonged expiratory phase, but as they struggle more and more with increasing pressure, the expiration may not always come to an end before the hypoxia and air hunger drives the next breath. The patient is not yet finished breathing out, when they are triggered by their brains to take a new breath in, and more air is inevitably trapped.
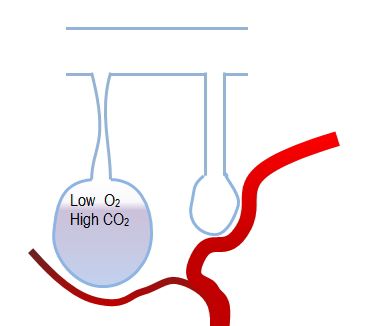
Obstructed lungs at end of expiration
The trick comes in here, that even if we were able to (and we are to an extent), decrease how hard the patient has to work to move air (by increasing the external pressure of the air that moves into the lungs by blowing it in under pressure), this doesn’t really solve the problem. It only buys a little time, after which; the pressure inside the thorax will still be larger (due to air being blown in and still trapped by the bronchoconstriction) than the external pressure.
The pressure being used to assist the patient will need to be increased again to allow for some air movement. This can be only be done to a certain limit, as the chest wall can only expand to a point. Once the pressure driving the air in reaches equilibrium with the pressure trapped, there is no way that air can move in the lungs. This is when the patient often arrests or ends up with a “Silent Chest” when there is not enough pressure generated to move air into the lungs.
These patients arrest for a few reasons:
- The lungs are not able to allow for any movement of oxygen-rich air into the space as the pressure inside may be greater than the pressure outside the lungs, therefore hypoxia is a major contributor. The only way to overcome this is to increase the FiO2.
- The increased pressure in the chest, leads to decreased venous return (dynamic hyperinflation), as the pressure in the inferior vena cava is very low. It doesn’t take much extra intrathoracic pressure to drop the venous return, leaving the heart empty, and unable to push any blood to the lungs or body. The patient presents with a form of obstructive shock.
- Smaller airways collapse during expiration, trapping even more gas as the tiny airways just before the alveoli do not contain any cartilage and are made only of muscle, which under the extreme exertion of attempting to expire, can easily collapse just because the patient tries to force air out of their lungs. This means the above two problems are worsened.
- Hypercapnia (as well as the hypoxia described above) sets in and the patient starts to lose consciousness, resulting in a decrease in the ability to breathe both in and out. The extreme activity of trying to force air out as well as create a negative pressure to suck air in causes the movement of air to cease.
The image to the right shows the effect of massive expiratory pressure on the smaller airways, resulting in collapse, leading to an increase in trapping:
As if all the other stuff we have already covered is not enough… We also need to be careful about ending up in a situation where hyperoxia occurs (where we administer too much oxygen). This is a problem as the hypoxic vasoconstriction in the pulmonary circuit actually helps to maximize perfusion to the areas that are better ventilated and will begin to decrease if there is too much oxygen administered, and resulting in a worsening V/Q mismatch.
If all we are going to do is intubate the patient so we can force air under greater pressure into the lungs, we are not helping the patient at all. If your vent settings are such that we can minimise the pressure, and maximize the expiration, whilst allowing the medication we are still administering time to work, then we will be able to actually assist this patient.
Right…so we are at the point where we need to intubate and ventilate this patient… HELP!
Some things we have to get right from the start:
- The largest size ETT we can place in the airway is the one that should be placed. Increasing the size of the ETT decreases the pressure against which the patient has to breathe to get the air out.
- Delayed Sequence Intubation might be needed especially for the patient who is combative (using Ketamine in high doses will allow control of the patient while you try to better oxygenate the patient before intubation)
Ventilator Settings:
- Volume control modes of ventilation may be safer for this patient, as a controlled tidal volume can be administered, and even if there are dynamic changes in the compliance of the lungs then a safe tidal volume will be administered). --See the post on ventilation for more information on this point.
- Select a safe tidal volume (lung protective), this usually means anywhere from 5-7ml/kg-the higher the tidal volume, the higher the pressure will go as more air will need to flow through the same space leading to increases in pressure
- Set PEEP that is reasonable (this DOESN’T MEAN ZERO)-set a PEEP that will be low enough not to add to the pressure in the thorax, but high enough that it might help to splint the smaller airways open against the forced expiration-Anywhere from 2-6cmH20 should be safe, start lowish and stay low and see how the patient does-There is some evidence that in the paralyzed patient, PEEP may not add any positive effect (as there is no work of breathing and no forced expiration), but in the patient who is spontaneously breathing, adding low levels of PEEP may be beneficial to decrease work of breathing and fight against the collapse of the smaller airways on forced expiration (Laher and Buchanan, 2018).
- Decrease the respiratory rate to around 10-12 for an adult patient (for paeds it gets a bit tricky, but perhaps lower than normal)-This one seems counter intuitive, but at this point we want as long a time as possible for expiration, and this will assist with minimizing pressure build up in the chest-Decreasing the respiratory rate is MUCH more powerful in terms of increasing expiratory time than using the I:E ratio.
- Think about setting a higher than normal I:E ratio once the respiratory rate is set, this will be 1:3 or 1:4-This will maximize the expiratory time a little further, but wait and see if the reduced rate works to fix the problem
- Try to maintain the following pressures in safe parameters:-Plateau pressure should not exceed 25cmH20 – 30cmH20. Remember this is the pressure applied to the elastic airways or alveoli, and shouldn’t be too high as the air can’t really get there easily.-Peak pressures are going to alarm (this is the pressure applied to the conducting airways), and although we usually don’t want this to go above 35cmH20, it likely will. Set the high pressure alarm higher than usual and monitor it as you may need to go as high as 50-70cmH20 or even higher to achieve a tidal volume that meets the patients minimum requirement.-Call for help early in the ventilation of these patients
- Think about paralyzing the patient in the early stages to gain control of the pressure in the thorax and to get the patient not to breathe too fast-This patient will be air hungry, hypoxic and hypercapnic, and will likely want to breathe at a much faster rate than the 10-12b/min you set, this will be counter-productive as the faster the patient breathes the more pressure is trapped in the lungs.-Paralyze the patient with a longer acting paralytic to better control their ventilation
Anticipate that there will be a MUCH higher PaCO2 level than you might be comfortable with as a result of the slower respiratory rate and lower tidal volume. This will have to be accepted for the period whilst the patient is still trapping pressure. Permissive hypercapnia is the term used to describe this where you may have to accept PaCO2 levels that are very high. Provided the pH doesn’t drop below 7.2, this is considered to be safe.
If the patient’s condition is complicated by RICP (or some other disease process where CO2 level maintenance should be within strict norms) then this is not the best approach to take.
Affecting Expiratory Time
Some things we have to get right from the start:
Normal ventilation of a patient. All the settings on the right and all the monitored results in the grey block. Take note of the peak pressure, the I:E ratio and the inspiratory time.
When we adjust just the I:E time by decreasing the inspiratory time, we adjust the I:E ratio. BUT the actual expiratory time has only increased by 0.4 of second. Also notice what happened to the PIP when we made the inspiratory time shorter.
References
- Kenny, J. 2019. ICU Physiology in 1000 Words: Asthmatic Mechanics. Pulm CCM.
- Laher AE, Buchanan SK: Mechanically ventilating the severe asthmatic. Journal of intensive care medicine 2018, 33(9):491-501
- Rezaie, S. 2015. REBEL Cast Episode 11: The Crashing Asthmatic. REBEL EM blog, June 1, 2015.
- Yartsev, A. 2019. Ventilation strategies for Status Asthmaticus. Deranged Physiology.